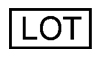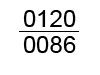Quality Certifications
Quality Policy
Modern Orthodontics strives to deliver a best in class customer experience by offering high quality orthodontic appliances on time, without defects and competitively priced.
Our dedicated team of professionals are trained to maintain and continually improve the effectiveness of our quality management system while ensuring compliance with applicable standards, regulatory requirements and customer requirements.
This will give our customer base the confidence that they will receive products that are of consistently high quality, with the added assurance that their individual needs and expectations will be met.
Quality System Management
MO's quality management system is certified by Underwriters Laboratories (UL) to the ISO 13485:2016 and standards to ensure consistent quality and high customer satisfaction. A quality management system is a common sense, well-documented system that ensures consistency and improvement of working practices, including the products and services produced. Quality management systems are based on standards, which specify a procedure for achieving effective quality management.
ISO standards contribute to making the development, manufacturing and supply of products and services more efficient, safer and cleaner. They make trade between countries easier and fairer. They provide governments with a technical base for health, safety and environmental legislation. ISO standards also serve to safeguard consumers, and users in general, of products and services.
ISO 13485:2016
Effective Quality Management Systems are recognized as a key regulatory consideration for allowing medical device manufacturers to market their products around the world. Whatever devices they produce, medical device manufacturers have a responsibility to consistently deliver devices that are safe and effective. ISO 13485:2016 is the international standard recognized for medical device Quality Management Systems registration.
ISO 13485:2016 provides a good base model for compliance with the EU CE marking Medical Devices Directives (Annex II, V, VI), Japan Pal and Health Canada CMDCAS (class II, III & IV devices) requirements. ISO 13485:2016 is also considered to be fully compatible with the FDA QSR.
View MO's ISO 13485:2016 certificate
CE Mark for EU Member Countries
MO's quality management has been certified to CE by UL. The CE mark is the official marking required by the European Community. CE Marking is often referred to as a 'passport' that allows manufacturers from anywhere in the world to sell their goods throughout the European market. In many countries within Europe, where a New Approach Directive is in force, CE Marking is a legal requirement for products, packaging and any accompanying literature. It proves to the buyer or user that the product fulfills all essential safety and environmental requirements as defined in the European Medical Device Directives.
View MO's CE certificate
Food and Drug Administration (FDA)
The FDA's mission is to promote and protect the public health by helping safe and effective products reach the market in a timely way, and monitoring products for continued safety after they are in use. The FDA is a blending law and science aimed at protecting consumers. MO's quality management system is compliant with the FDA's quality system regulation 21 CFR Parts 808, 812, and 820, Medical Devices; Current Good Manufacturing Practice (cGMP).
Modern Orthodontics is registered with FDA as a manufacturer.
View MO's FDA registration status
Material Safety Data Sheets
In 1988, the Department of Labor passed the Occupational Safety and Health Act (OSHA). In order to ensure chemical safety in the workplace, information must be available about the identities and hazards of the chemicals used in the workplace. OSHA's Hazard Communication Standard (HCS) requires the development and dissemination of such information through Material Safety Data Sheets.
View the MO's Material Safety Data Sheets
Product Label Symbols
The information provided below is an explanation of reference symbols that can be found on product labels.
Catalog Number / "Reference" This symbol immediately precedes the part number of the product.
LOT Batch Code / "Lot" This symbol immediately precedes the lot number of the product. MO uses this for part traceability.
Authorised Representative in the European Community / "EU Rep" This symbol immediately precedes the name of our representative within the member states.
Do Not Reuse / "Single Use Only" Devices with this symbol are intended for single use.
Hourglass Use By / "Hourglass" This symbol identifies that the part has a "Use by" date. The part should be used prior to the date located next to this symbol. Date format YYYY-MM, i.e. 2013-11.
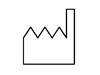
Manufacturer / "Filled Factory" Symbol for Manufacturer. May also include the date of manufacture in date format YYYY-MM.
Manufactured Date / "Open Factory" Date of manufacture listed under or beside this symbol in YYYY-MM format. Note: Used only when the "filled factory" symbol is not present.
Consult Instructions for Use / "IFU Book" For products with additional instructions inside the package.
"Non-Sterile Triangle" Not Used: G&H products are sold non-sterile.
Keep away from Sunlight / "Sunlight Warning" Used on elastics. Product may demonstrate reduced performance / longevity when exposed to sunlight.
Contains latex or Presence of Natural Rubber Latex / "Latex Warning" Used on latex elastics.
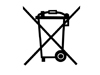
Trash Do Not Dispose in Household Trash Used for products with batteries or electrical components, cords, chargers, etc.
Nickel and/or Chromium warning Caution: Product contains Nickel and/or Chromium. To alert of the potential for allergic reactions to these known allergens.
The Safe Drinking Water and Toxic Enforcement Act of 1986 / "Proposition 65" This product contains chemicals known to the State of California to cause cancer and other birth defects.
Rx Only For use or distribution by orthodontic professionals only.
HooksSymbol used to identify brackets that have a hook.
Datmatrix / "Barcode" Manufacturer code use to contain traceability data of the particular part.
CE Mark European Conformance to Medical Device Directive 93/42/EEC
CE Class II Used with the CE mark, the numbers provide eference to our Notified Body. For Indiana, 0120 is used; for California, 0086 is used.
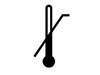
Temperature Used to denote appropriate storage temperatures, when controlled storage is required. Numbers to the left of the symbol are min, or the lowest temperature used in the storage of the device, numbers to the right are max, or the highest temperature used in the storage of the device.
Warning See additional symbols / Instructions for use.
Sharp Caution: package contains sharp object.
This is our company logo which appears on all our products.